Thermo-Mechanical Analysis of Brain Tissue During Freezing
-
摘要:
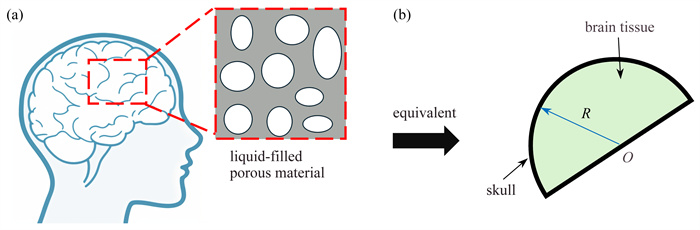
虽然大脑是人体最重要的器官,但其在低温冷冻过程中的热-力耦合机理仍不明晰. 该文考虑颅脑特殊形状、多孔弹性、脑脊液流动、颅骨约束以及冻胀效应,建立脑组织低温冷冻热-力耦合模型,通过分析冷冻过程中的温度场、相场和脑脊液冻胀产生的压力场,发现在凝固过程中脑脊液温度保持不变,而脑组织内部最大温差可达20 K. 固-液相界面厚度约0.3 mm,推进速度约0.09 mm/s. 冻胀产生的脑组织最大位移(~0.12 mm)发生在靠近头盖骨处. 固液界面处压力梯度高达500 MPa/mm,而固体和脑脊液内部压力几乎不变. 本研究可为人类大脑的低温冷冻保存策略及脑防护提供理论支撑.
Abstract:Although the brain is the most important organ in the human body, its thermo-mechanical coupling mechanism during cryogenic freezing remains unclear. A thermo-mechanical model for the cryogenic freezing of brain tissue was established, considering the special shape of the skull and brain, the cerebrospinal fluid, the cranial constraints, and the frost-heave effects. Analyses of the temperature field, the phase field, and the pressure field caused by the frost heave of the cerebrospinal fluid during freezing show that, the temperature of the cerebrospinal fluid remains unchanged during coagulation, while the maximum temperature difference within the brain tissue could reach 20 K. The solid-liquid phase interface is about 0.3 mm thick, and the driving velocity is about 0.09 mm/s. The maximum displacement of the brain tissue due to freezing is about 0.12 mm near the skull, and the pressure gradient at the solid-liquid interface is as high as 500 MPa/mm, while the pressure inside the solid and the CSF keeps almost unchanged. This study provides a theoretical support for the human brain cryopreservation strategy and the brain protection.
-
Key words:
- brain tissue /
- cerebrospinal fluid /
- poro-elasticity /
- frost heaving pressure
edited-byedited-by1) (我刊编委刘少宝来稿) -
表 1 脑组织物理参数取值
Table 1. Values of brain physical parameters
physical parameter value range reference value size ri,ro 5 cm thermal conductivity of matrix λm grey matter 0.57 W/(m·K)[22]
white matter 0.50 W/(m·K)[22]
brain tissue 0.66 W/(m·K)[23]0.53 W/(m·K) thermal conductivity of water λw cerebrospinal fluid 0.62 W/(m·K)[22]
plasma 0.63 W/(m·K)[22]
blood 0.63 W/(m·K)[23]
water 0.59 W/(m·K)[24]0.60 W/(m·K) thermal conductivity of ice λi ice 2.1 W/(m·K)[25] 2.1 W/(m·K) specific heat capacity of matrix cm grey matter 3.7 kJ/(kg·K)[22]
white matter 3.6 kJ/(kg·K)[22]3.7 kJ/(kg·K) specific heat capacity of water cw cerebrospinal fluid 4.2 kJ/(kg·K)[22]
blood 3.6 W/(m3·K)[23]
water 4.2 kJ/(kg·K)[26]4.2 kJ/(kg·K) specific heat capacity of ice ci ice 2.1 kJ/(kg·K)[25] 2.1 kJ/(kg·K) density of matrix ρm grey matter 1 038 g/cm3[22]
white matter 1 039 g/cm3[22]1.038 g/cm3 density of water ρw cerebrospinal fluid 1 007 kg/m3[22]
blood 1 050 kg/m3[23]1 007 kg/m3 density of ice ρi ice 917 kg/m3[27] 900 kg/cm3 Young’s modulus of brain Em 338.15 Pa[28] 338.15 Pa Young’s modulus of ice Ei 8 GPa[29] 8 GPa bulk modulus of water Kw 2 GPa 2 GPa Poisson’s ratio of brain μm 0.3[28] 0.3 phase transition temperature of water Tf 273 K[30] 273 K latent heat of phase change L 334 kJ/kg[31] 334 kJ/kg environment temperature Te 253 K[32] 253 K initial temperature Ti 293 K saturation capacity θs 0.75~0.95[6] 0.9 -
[1] ONODY R N. Criptobiose: um Vermeressuscitadepois de 46.000 Anos[M]. São Carlos: Universidade de São Paulo, 2023. [2] MORRIS G J, ACTON E, MURRAY B J, et al. Freezing injury: the special case of the sperm cell[J]. Cryobiology, 2012, 64(2): 71-80. doi: 10.1016/j.cryobiol.2011.12.002 [3] JANG T H, PARK S C, YANG J H, et al. Cryopreservation and its clinical applications[J]. Integrative Medicine Research, 2017, 6(1): 12-18. doi: 10.1016/j.imr.2016.12.001 [4] KARLSSON J O, TONER M. Long-term storage of tissues by cryopreservation: critical issues[J]. Biomaterials, 1996, 17(3): 243-256. doi: 10.1016/0142-9612(96)85562-1 [5] ZHANG M, LI F, DIAO X, et al. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles[J]. Meat Science, 2017, 133: 10-18. doi: 10.1016/j.meatsci.2017.05.019 [6] KUMARASAMI R, VERMA R, PANDURANGAN K, et al. A technology platform for standardized cryoprotection and freezing of large-volume brain tissues for high-resolution histology[J]. Frontiers in Neuroanatomy, 2023, 17: 1292655. doi: 10.3389/fnana.2023.1292655 [7] CONAWAY R M. Method for preservation and storage of viable biological materials at cryogenic temperatures[P]. 1987-08-25. [8] HUNT C J. Technical considerations in the freezing, low-temperature storage and thawing of stem cells for cellular therapies[J]. Transfusion Medicine and Hemotherapy, 2019, 46(3): 134-150. doi: 10.1159/000497289 [9] KUMAR R, MOHANARAO G J, ATREJA A S K. Freeze-thaw induced genotoxicity in buffalo (bubalus bubalis) spermatozoa in relation to total antioxidant status[J]. Molecular Biology Reports, 2011, 38: 1499-1506. doi: 10.1007/s11033-010-0257-1 [10] LEE R E, ALLENSPACH A L, COLLINS S D. Ultrastructural effects of lethal freezing on brain, muscle and Malpighian tubules from freeze-tolerant larvae of the gall fly, Eurostasolidaginis[J]. Journal of Insect Physiology, 1997, 43(1): 39-45. doi: 10.1016/S0022-1910(96)00073-X [11] PEGG D E. The relevance of ice crystal formation for the cryopreservation of tissues and organs[J]. Cryobiology, 2010, 60(3): S36-S44. doi: 10.1016/j.cryobiol.2010.02.003 [12] KLOCKE S, BVNDGEN N, KÖSTER F, et al. Slow-freezing versus vitrification for human ovarian tissue cryopreservation[J]. Archives of Gynecology and Obstetrics, 2015, 291: 419-426. doi: 10.1007/s00404-014-3390-6 [13] NICOLAS G. Advantages of fast-freeze fixation followed by freeze-substitution for the preservation of cell integrity[J]. Journal of Electron Microscopy Technique, 1991, 18(4): 395-405. doi: 10.1002/jemt.1060180408 [14] DE GRAAF I, VAN DER VOORT D, et al. Increased post-thaw viability and phase Ⅰ and Ⅱ biotransformation activity in cryopreserved rat liver slices after improvement of a fast-freezing method[J]. Drug Metabolism and Disposition, 2000, 28(9): 1100-1106. [15] HUNT C J. Cryopreservation: vitrification and controlled rate cooling[J]. Methods in Molecular Biology, 2017, 1590: 41-77. [16] PINSKIY V, TOLPYGO A S, JONES J, et al. A low-cost technique to cryo-protect and freeze rodent brains, precisely aligned to stereotaxic coordinates for whole-brain cryosectioning[J]. Journal of Neuroscience Methods, 2013, 218(2): 206-213. doi: 10.1016/j.jneumeth.2013.03.004 [17] ROSENE D L, ROY N J, DAVIS B J. A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact[J]. Journal of Histochemistry & Cytochemistry, 1986, 34(10): 1301-1315. [18] CHOWDHURY F, HUANG B, WANG N. Cytoskeletal prestress: the cellular hallmark in mechanobiology and mechanomedicine[J]. Cytoskeleton, 2021, 78(6): 249-276. doi: 10.1002/cm.21658 [19] ZHANG J, REINHART-KING C A. Targeting tissue stiffness in metastasis: mechanomedicine improves cancer therapy[J]. Cancer Cell, 2020, 37(6): 754-755. doi: 10.1016/j.ccell.2020.05.011 [20] 季葆华. 生命系统中的力化耦合定量机制与力医学路径初探[J]. 医用生物力学, 2023, 38(3): 433-450. https://www.cnki.com.cn/Article/CJFDTOTAL-YISX202303003.htmJI Baohua. Mechano-chemical coupling in living organisms and possible road map of mechanomedicine[J]. Journal of Medical Biomechanics, 2023, 38(3): 433-450. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-YISX202303003.htm [21] 郭卉, 贺昱昇, 刘梦洁, 等. 肿瘤力医学[J]. 中华肿瘤杂志, 2024, 46(6): 536-548.GUO Hui, HE Yusheng, LIU Mengjie, et al. Tumor mechanomedicine[J]. Chinese Journal of Oncology, 2024, 46(6): 536-548. (in Chinese) [22] SCHOONEVELDT G, TREFNÁ H D, PERSSON M, et al. Hyperthermia treatment planning including convective flow in cerebrospinal fluid for brain tumour hyperthermia treatment using a novel dedicated paediatric brain applicator[J]. Cancers, 2019, 11(8): 1183. doi: 10.3390/cancers11081183 [23] PONDER E. The coefficient of thermal conductivity of blood and of various tissues[J]. The Journal of General Physiology, 1962, 45(3): 545-551. doi: 10.1085/jgp.45.3.545 [24] KELL G, WHALLEY E. Reanalysis of the density of liquid water in the range 0~150 ℃ and 0~1 kbar[J]. The Journal of Chemical Physics, 1975, 62(9): 3496-3503. doi: 10.1063/1.430986 [25] MYERS T, LOW J. An approximate mathematical model for solidification of a flowing liquid in a microchannel[J]. Microfluidics and Nanofluidics, 2011, 11: 417-428. doi: 10.1007/s10404-011-0807-4 [26] MANYA J J, ANTAL JR M J, KINOSHITA C K, et al. Specific heat capacity of pure water at 4. 0 MPa between 298. 15 and 465. 65 K[J]. Industrial & Engineering Chemistry Research, 2011, 50(10): 6470-6484. [27] PUSTOGVAR A, KULYAKHTIN A. Sea ice density measurements. Methods and uncertainties[J]. Cold Regions Science and Technology, 2016, 131: 46-52. doi: 10.1016/j.coldregions.2016.09.001 [28] SU L, WANG M, YIN J, et al. Distinguishing poroelasticity and viscoelasticity of brain tissue with time scale[J]. Acta Biomaterialia, 2023, 155: 423-435. doi: 10.1016/j.actbio.2022.11.009 [29] GOW A J, UEDA H T, GOVONI J W, et al. Temperature and structure dependence of the flexural strength and modulus of freshwater model ice: CRREL Rept 88-6[R]. 1988. [30] WAN X, LIU E, QIU E. Study on ice nucleation temperature and water freezing in saline soils[J]. Permafrost and Periglacial Processes, 2021, 32(1): 119-138. doi: 10.1002/ppp.2081 [31] JAIN A, MIGLANI A, HUANG Y, et al. Ice formation modes during flow freezing in a small cylindrical channel[J]. International Journal of Heat and Mass Transfer, 2019, 128: 836-848. doi: 10.1016/j.ijheatmasstransfer.2018.08.051 [32] SCHÄFER A T, KAUFMANN J D. What happens in freezing bodies? Experimental study of histological tissue change caused by freezing injuries[J]. Forensic Science International, 1999, 102(2/3): 149-158. -





 下载:
下载:





 渝公网安备50010802005915号
渝公网安备50010802005915号