Effects of Cartilage Endplate Degeneration on Metabolic Transport and Biomechanical Responses of Cervical Intervertebral Discs
-
摘要:
软骨终板内的液体流动是椎间盘营养供给和代谢废物运输的主要途径. 退化的终板刚度增加、渗透性和含水量下降,会影响椎间盘内物质运输和力学响应. 基于人体颈椎计算机断层扫描数据建立了C5-C6节段的多孔介质有限元模型. 对验证后的模型施加压缩、前屈、后伸、轴向旋转和侧弯五种载荷,通过改变终板渗透性、孔隙比和模量,分析了正常、钙化和硬化三种状态下椎间盘的响应. 结果表明:软骨终板退化增加了软骨终板和髓核的多孔压力,降低了软骨终板的流体速度. 前屈载荷下,与正常终板对比,钙化和硬化终板导致髓核内流体的多孔压力分别增加了50.8%和88.9%. 退化终板渗透率和含水量的降低导致髓核内液体不易流动,增加了髓核基体的应力,在压缩和轴向旋转载荷下,硬化终板导致髓核基体的最大主应力分别增加了122.2%和100.0%.
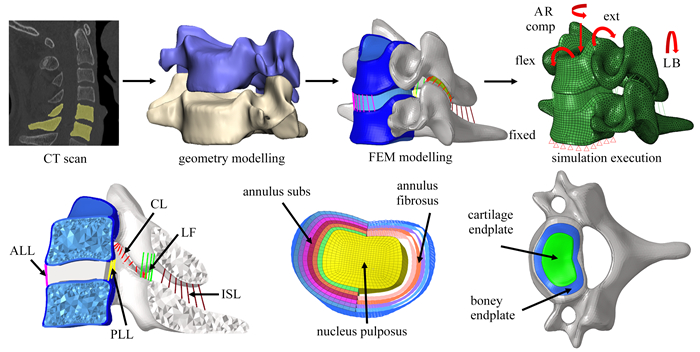
Abstract:The fluid flow in the cartilage endplate (CEP) is the main path of nutrient supply and metabolic waste transport within the intervertebral disc (IVD). The increased stiffness, the decreased permeability and the water content of the degenerated cartilage endplate influence the mechanical responses and material transport within the IVD. A porous finite element model for C5-C6 of the cervical spine was established based on the computed tomography (CT) images of an adult. After validation, loads of compression, flexion, extension, axial rotation and lateral bending were applied to this model to calculate the instantaneous responses of the IVD. The calcification and sclerosis in the CEP were simulated with increase of its modulus and decrease of its permeability and porosity, compared with a healthy case. The results show that, the pore pressures within the CEP and the nucleus pulposus (NP) increase and the fluid velocity decrease in the degenerated CEP. Under flexion, the pore pressure in the NP increase by 50.8% and 88.9% in calcified and sclerotic CEPs compared to the healthy endplate, respectively. The decreases of the permeability and the water content in the degenerated CEP hinder the fluid flow and increase the maximum principal stresses of the NP matrix by 122.2% and 100.0% under compression and axial rotation, respectively.
-
表 1 多孔介质有限元模型材料参数
Table 1. Material parameters of the porous finite element model
component Young’s modulus E/MPa Poisson’s ratio ν void ratio e0 permeability k0/(mm4/(N·s)) reference cortical bone 16 800 0.3 - - [23] cancellous bone 450 0.3 0.41 5.773 5E-2 [16, 24] posterior bone 3 500 0.3 - - [24] articular cartilage 10 0.4 - - [19] nucleus pulposus 1 0.49 5.67 1.56E-4 [16, 24] annulus substance Mooney-Rivlin
C10=0.133,
C01=0.033 3, D1=0.62.45 1.56E-4 [16, 25] annulus fibrosus 110 0.3 - - [25] boney endplate 5 600 0.3 0.8 7.500E-2 [13, 19] cartilage endplate healthy calcified sclerotic 5
3 5000.4 4
0.114.041E-3
4.041E-4
4.041E-5[16, 24]
[13, 26]ligament ALL 28.2 0.4 - - [27-28] PLL 23 0.4 LF 3.5 0.4 CL 4.8 0.4 ISL 5 0.4 表 2 髓核及软骨终板在压缩、前屈、后伸、轴向旋转及侧弯载荷下的最大多孔压力比较
Table 2. Comparison of maximum pore pressures in the nucleus pulposus and the cartilage endplate under compression (comp), flexion (flex), extension (ext), axial rotation (AR) and lateral bending (LB)
component degree of cartilage endplate degeneration loading condition comp flex ext AR LB nucleus pulposus calcified 12.5% 50.8% 1.4% 14.3% 1.5% sclerotic 55.0% 88.9% 1.4% 48.2% 19.7% cartilage endplate calcified 95.7% 97.9% 60.0% 42.2% 38.6% sclerotic 169.6% 147.9% 80.0% 84.4% 56.1% 表 3 髓核及软骨终板在压缩、前屈、后伸、轴向旋转及侧弯载荷下的最大流体速度比较
Table 3. Comparison of maximum fluid velocities in the nucleus pulposus and the cartilage endplate under compression (comp), flexion (flex), extension (ext), axial rotation (AR) and lateral bending (LB)
component degree of cartilage endplate degeneration loading condition comp flex ext AR LB nucleus pulposus calcified -29.1% 9.5% -8.6% 0.7% -1.3% sclerotic -19.4% 46.7% 2.6% 15.0% 14.6% cartilage endplate calcified -32.3% -19.5% -70.0% -60.9% -67.8% sclerotic -89.3% -89.5% -96.4% -94.5% -95.4% 表 4 髓核及软骨终板在压缩、前屈、后伸、轴向旋转及侧弯载荷下的最大主应力比较
Table 4. Comparison of maximum principal stresses in the nucleus pulposus and the cartilage endplate under compression (comp), flexion (flex), extension (ext), axial rotation (AR) and lateral bending (LB)
component degree of cartilage endplate degeneration loading condition comp flex ext AR LB nucleus pulposus calcified 77.8% 70.6% 12.5% 69.2% 31.3% sclerotic 122.2% 94.1% 43.8% 100.0% 56.3% cartilage endplate calcified 330.0% 390.6% 571.4% 325.0% 492.3% sclerotic 335.0% 384.4% 595.4% 320.8% 480.8% -
[1] SAFIRI S, KOLAHI A A, HOY D. Global, regional, and national burden of neck pain in the general population, 1990-2017: systematic analysis of the global burden of disease study 2017[J]. BMJ British Medical Journal, 2020, 368: m791. [2] KAZEMINASAB S, NEJADGHADERI S A, AMIRI P, et al. Neck pain: global epidemiology, trends and risk factors[J]. BMC Musculoskeletal Disorders, 2022, 23(1): 26. doi: 10.1186/s12891-021-04957-4 [3] HUANG H W, LIU J L, WANG L Z, et al. A critical review on the biomechanical study of cervical interbody fusion cage[J]. Medicine in Novel Technology and Devices, 2021, 11: 100070. doi: 10.1016/j.medntd.2021.100070 [4] CRUMP K B, ALMINNAWI A, BERMUDEZ-LEKERIKA P, et al. Cartilaginous endplates: a comprehensive review on a neglected structure in intervertebral disc research[J]. JOR Spine, 2023, 6(4): e1294. doi: 10.1002/jsp2.1294 [5] WILLS C R, FOATA B, BALLESTER M A G, et al. Theoretical explorations generate new hypotheses about the role of the cartilage endplate in early intervertebral disk degeneration[J]. Frontiers in Physiology, 2018, 9: 1210. doi: 10.3389/fphys.2018.01210 [6] WONG J, SAMPSON S L, BELL-BRIONES H, et al. Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy[J]. Osteoarthritis and Cartilage, 2019, 27(6): 956-964. doi: 10.1016/j.joca.2019.01.013 [7] ANTONIOU J, STEFFEN T, NELSON F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration[J]. Journal of Clinical Investigation, 1996, 98(4): 996-1003. doi: 10.1172/JCI118884 [8] GRANT M P, EPURE L M, BOKHARI R, et al. Human cartilaginous endplate degeneration is induced by calcium and the extracellular calcium-sensing receptor in the intervertebral disc[J]. European Cells & Materials, 2016, 32: 137-151. [9] REN P, CHEN P, REEVES R A, et al. Diffusivity of human cartilage endplates in healthy and degenerated intervertebral disks[J]. Journal of Biomechanical Engineering-Transactions of the ASME, 2023, 145(7): 071006. doi: 10.1115/1.4056871 [10] SAMPSON S L, SYLVIA M, FIELDS A J. Effects of dynamic loading on solute transport through the human cartilage endplate[J]. Journal of Biomechanics, 2019, 83: 273-279. doi: 10.1016/j.jbiomech.2018.12.004 [11] BIAN Q, JAIN A, XU X, et al. Excessive activation of TGFβ by spinal instability causes vertebral endplate sclerosis[J]. Scientific Reports, 2016, 6: 27093. doi: 10.1038/srep27093 [12] LIU Q, YANG Z, LIU Y, et al. Cervical spinal instability causes vertebral microarchitecture change and vertebral endplate lesion in rats[J]. Journal of Orthopaedic Translation, 2020, 24: 209-217. doi: 10.1016/j.jot.2019.10.005 [13] HASSAN C R, LEE W, KOMATSU D E, et al. Evaluation of nucleus pulposus fluid velocity and pressure alteration induced by cartilage endplate sclerosis using a poro-elastic finite element analysis[J]. Biomechanics and Modeling in Mechanobiology, 2021, 20(1): 281-291. doi: 10.1007/s10237-020-01383-8 [14] 李树光, 曲凯. 多孔介质中单相气体局部流动的均质化建模[J]. 应用数学和力学, 2024, 45(2): 175-183. doi: 10.21656/1000-0887.440246LI Shuguang, QU Kai. Homogenization modeling of single-phase gas local flow in porous media[J]. Applied Mathematics and Mechanics, 2024, 45(2): 175-183. (in Chinese) doi: 10.21656/1000-0887.440246 [15] 何树, 娄钦. 多孔介质孔隙率对池沸腾传热性能影响机理的模拟研究[J]. 应用数学和力学, 2024, 45(3): 348-364. doi: 10.21656/1000-0887.440212HE Shu, LOU Qin. Simulation study of porosity effects of porous media on pool boiling heat transfer performances[J]. Applied Mathematics and Mechanics, 2024, 45(3): 348-364. (in Chinese) doi: 10.21656/1000-0887.440212 [16] HUSSAIN M, NATARAJAN R N, AN H S, et al. Progressive disc degeneration at C5-C6 segment affects the mechanics between disc heights and posterior facets above and below the degenerated segment: a flexion-extension investigation using a poroelastic C3-T1 finite element model[J]. Medical Engineering & Physics, 2012, 34(5): 552-558. [17] HUSSAIN M, NATARAJAN R N, CHAUDHARY G, et al. Relative contributions of strain-dependent permeability and fixed charged density of proteoglycans in predicting cervical disc biomechanics: a poroelastic C5-C6 finite element model study[J]. Medical Engineering and Physics, 2011, 33(4): 438-445. doi: 10.1016/j.medengphy.2010.11.011 [18] GUO L X, LI R, ZHANG M. Biomechanical and fluid flowing characteristics of intervertebral disc of lumbar spine predicted by poroelastic finite element method[J]. Acta of Bioengineering and Biomechanics, 2016, 18(2): 19-29. [19] PANZER M B, CRONIN D S. C4-C5 segment finite element model development, validation, and load-sharing investigation[J]. Journal of Biomechanics, 2009, 42(4): 480-490. doi: 10.1016/j.jbiomech.2008.11.036 [20] NIKKHOO M, HSU Y C, HAGHPANAHI M, et al. A meta-model analysis of a finite element simulation for defining poroelastic properties of intervertebral discs[J]. Proceedings of the Institution of Mechanical Engineers(Part H): Journal of Engineering in Medicine, 2013, 227(6): 672-682. doi: 10.1177/0954411913480668 [21] KOJIC M, FILIPOVIC N, VULOVIC S, et al. A finite element solution procedure for porous medium with fluid flow and electromechanical coupling[J]. Communications in Numerical Methods in Engineering, 1998, 14(4): 381-392. doi: 10.1002/(SICI)1099-0887(199804)14:4<381::AID-CNM157>3.0.CO;2-1 [22] ARGOUBI M, SHIRAZI-ADL A. Poroelastic creep response analysis of a lumbar motion segment in compression[J]. Journal of Biomechanics, 1996, 29(10): 1331-1339. doi: 10.1016/0021-9290(96)00035-8 [23] REILLY D T, BURSTEIN A H, FRANKEL V H. The elastic modulus for bone[J]. Journal of Biomechanics, 1974, 7(3): 271-275. doi: 10.1016/0021-9290(74)90018-9 [24] CAI X Y, SANG D C, YUCHI C X, et al. Using finite element analysis to determine effects of the motion loading method on facet joint forces after cervical disc degeneration[J]. Computers in Biology and Medicine, 2020, 116: 103519. doi: 10.1016/j.compbiomed.2019.103519 [25] WANG K, DENG Z, WANG H H, et al. Influence of variations in stiffness of cervical ligaments on C5-C6 segment[J]. Journal of the Mechanical Behavior of Biomedical Materials, 2017, 72: 129-137. doi: 10.1016/j.jmbbm.2017.05.005 [26] GALBUSERA F, SCHMIDT H, NEIDLINGER-WILKE C, et al. The mechanical response of the lumbar spine to different combinations of disc degenerative changes investigated using randomized poroelastic finite element models[J]. European Spine Journal, 2011, 20(4): 563-571. doi: 10.1007/s00586-010-1586-4 [27] ZHOU E Z, HUANG H W, ZHAO Y B, et al. The effects of titanium mesh cage size on the biomechanical responses of cervical spine after anterior cervical corpectomy and fusion: a finite element study[J]. Clinical Biomechanics, 2022, 91: 105547. doi: 10.1016/j.clinbiomech.2021.105547 [28] MO Z J, LI Q, JIA Z W, et al. Biomechanical consideration of prosthesis selection in hybrid surgery for bi-level cervical disc degenerative diseases[J]. European Spine Journal, 2017, 26(4): 1181-1190. doi: 10.1007/s00586-016-4777-9 [29] SCHMIDT H, SHIRAZI-ADL A, GALBUSERA F, et al. Response analysis of the lumbar spine during regular daily activities: a finite element analysis[J]. Journal of Biomechanics, 2010, 43(10): 1849-1856. doi: 10.1016/j.jbiomech.2010.03.035 [30] HEUER F, SCHMITT H, SCHMIDT H, et al. Creep associated changes in intervertebral disc bulging obtained with a laser scanning device[J]. Clinical Biomechanics, 2007, 22(7): 737-744. doi: 10.1016/j.clinbiomech.2007.04.010 [31] ADAMS M A, HUTTON W C. The effect of posture on the fluid content of lumbar intervertebral discs[J]. Spine, 1983, 8(6): 665-671. doi: 10.1097/00007632-198309000-00013 [32] ADAMS M A, MCNALLY D S, DOLAN P. "Stress" distributions inside intervertebral discs[J]. Journal of Bone and Joint Surgery, 1996, 78(6): 965-972. doi: 10.1302/0301-620X.78B6.0780965 [33] FIELDS A J, BALLATORI A, LIEBENBERG E C, et al. Contribution of the endplates to disc degeneration[J]. Current Molecular Biology Reports, 2018, 4(4): 151-160. doi: 10.1007/s40610-018-0105-y -





 下载:
下载:







 渝公网安备50010802005915号
渝公网安备50010802005915号